Abstract
Background and aims
The extensive use of Venetoclax (VEN) in AML patients both in induction and in salvage therapy has evidenced critical issues in its day-to-day management. VEN, primarily metabolized by the cytochrome P450 (CyP3A4), has relevant known drug interactions. Therefore, dose adjustments have been suggested in the presence of CyP3A4 strong inhibitors as posaconazole (POS), used in primary antifungal prophylaxis. In the first cycle, VEN and POS start at different timepoints and it is not known how is the trend of their plasma concentrations. Therapeutic drug monitoring (TDM) of VEN could be a useful tool to define the drug exposure, but at present, reports on VEN TDM are few, with variable methods.
We designed a study on VEN plasma concentrations during ramp-up and after the introduction of POS through a high-performance liquid chromatography-tandem mass spectrometry (HPLC-MS/MS) to determine the steady state of VEN and the exposure between day 7 and day 12.
We here report the preliminary results obtained from 105 VEN plasma concentrations of 7 AML patients.
Methods
We collected 105 samples from 7 patients (F: 4/M: 3) with a median age of 73 years affected by AML, 2 de novo and 5 secondary (2 post myeloproliferative neoplasm, 2 post myelodysplasia and 1 post chronic myelomonocytic leukemia) during their first cycle of Azacytidine (Aza) or Decitabine (Dec) -VEN.
Treatment consisted in 28-day cycle with subcutaneous azacytidine (Aza) 75 mg/mq/day for 7 days or intravenous decitabine (Dec) 20 mg/mq for 5 days combined with a daily dose escalation of oral 100 mg (day 1), 200 mg (day 2), 400 mg (day 3) VEN, then reduced to 70 mg daily from day 4 when oral posaconazole (POS) 300 mg QD coadministration started. On days 7 and 12 we collected blood samples from each patient just before VEN administration and after 2, 4, 6 and 8 hours.
A rapid, sensitive and specific analytical method based on HPLC-MS/MS has been developed and validated. Both the analyte and the internal standard were detected after protein precipitation of small plasma sample with CH3OH:CH3CN = 50:50 containing IS. Elution was carried out in gradient mode (using a reverse phase chromatographic column). The detection was performed using a triple quadrupole mass spectrometer, in the positive electrospray ionization mode. The concentration time profiles and the area under the plasma concentration-time curve (AUC0-8h) were analyzed on both days 7 and 12. AUC0-24 was determined only on day 12, when it was considered that the steady state was achieved. The accumulation ratio was also estimated as ratio of the exposures on day 12 and 7.
Results
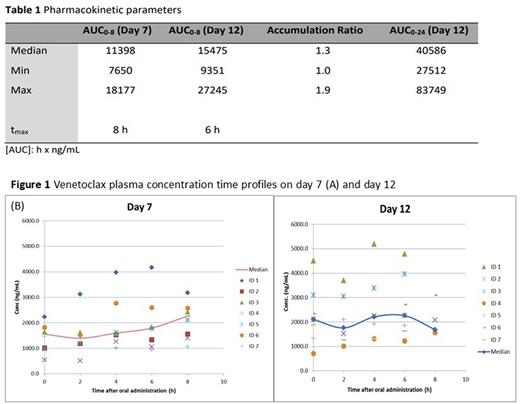
All available pharmacokinetic data are summarized in Table 1.
The inter-individual pharmacokinetic variability of VEN was large especially on day 7, when the steady state was not yet achieved (Fig. 1A). A continuous slight increase in concentration over the entire period of analysis (t0 - t8) was observed on day 7, while a maximum concentration (CMAX) was recognized 6 hours after drug administration on day 12 (Fig. 1B).
Median AUC0-8 were 11398 and 15475 hxng/mL on day 7 and 12 respectively, indicating VEN increase with a median accumulation ratio of 1.3. The calculated coefficient of variation in AUC0-8 was approximately 40% on both days (36 and 39% on day 7 and 12, respectively). The median 24-hour VEN exposure (AUC0-24) was 40586 hxng/ml, corresponding to a median steady state concentration (Css) of 1691 ng/mL.
Conclusions and Discussion
VEN plasma concentrations have been reported by several authors in a wide variability range. Values here determined are substantially comparable with literature data for cases treated without CyP3A4 inhibitors or at the dose of 50 mg with POS (3). Moreover, although concentrations progressively increase after seven days from the start of VEN and the introduction of POS, exposure at day 7 resulted quite consistent with the one at day 12 when steady state has been achieved, suggesting that at day 7 VEN was already at therapeutic levels. Finally, the 24-hour exposure at day 12 resulted comparable with values reported for VEN daily doses of 600 or 800 mg without CyP3A4 inhibitors (2), suggesting that the recommended dose of 70 mg if combined with POS could probably be reduced.
The validated analytical method has been shown rapid, sensitive, specific and reliable. Additional data are needed to confirm this trend of VEN values and to demonstrate the clinical utility of TDM of VEN used in combination with POS.
Disclosures
Arcaini:ADC Therapeutics: Membership on an entity's Board of Directors or advisory committees; Celgene/Bristol Myers Squibb: Membership on an entity's Board of Directors or advisory committees; EUSA Pharma: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Gilead Sciences: Research Funding; Incyte: Membership on an entity's Board of Directors or advisory committees; Janssen-Cilag: Membership on an entity's Board of Directors or advisory committees; Kite/Gilead: Membership on an entity's Board of Directors or advisory committees; Novartis: Speakers Bureau; Roche: Membership on an entity's Board of Directors or advisory committees; Verastem: Membership on an entity's Board of Directors or advisory committees.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal